Laboratory staff
Piotr Foltyński, PhD, DSc, Assoc. Prof. ( )
)
Piotr Ładyżyński, PhD, DSc, Prof. ( )
)
Janusz Krzymień, PhD, DSc, Assoc. Prof. ( )
)
Beata Toczyłowska, PhD, DSc, Assoc. Prof. ( )
)
Justyna Augustyniak, PhD ( )
)
Maria Molik, PhD
Paulina Duda, BSc
Selected current activity
- Seeking for new fields of application for the VoiceDiab system in type II diabetes, gestational diabetes and in persons with improper body weight.
- Studying relation between glycated hemoglobin A1c (HbA1c) concentration in blood and patient’s course of glycemia. Validation of mathematical models of HbA1c formation to predict changes of HbA1c concentration in response to varying glycemia courses. The experimental procedure uses an original combination of in vivo continuous glucose concentration monitoring, long-term in vitro culturing of the human erythrocytes and mathematical modeling of HbA1c formation in vivo and in vitro with HbA1c values scaled according to the most specific analytical methods.
- Development of new more accurate and precise methods of wound surface area measurement. As a result, the application AreaMe for Windows Mobile 6.5 system and a Planimator app for Android 4.0 were developed and tested. The Planimator app may be send to any user for the research purposes (contact e-mail: pfoltynski(at)ibib.waw.pl). The manual of the Planimator app is under the following link Planimator manual.pdf.
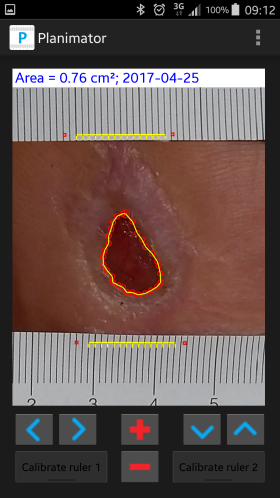
Sample screenshot of the Planimator app with displayed area of a measured wound at foot sole of a diabetic patient
- Development of a bioreactor (capillary module), which can simulate human blood vessel, with capillary polysulfone membranes covered with the human umbilical vein endothelial cells (HUVECs). Such artificial blood vessel may be used in experimental models in studies pathophysiological mechanisms of different diseases.

Endothelial cells at the inner surface of a single bioreactor capillary (left picture) and smooth muscle cells at the outer surface this capillary (right picture) after 48 h incubation in bioreactor
- Studying influence of zinc oxide nanoparticles on endothelial cells cultured in hollow fiber bioreactor under dynamic flow of growth medium.
- Metabolic profile analysis of biofluids and tissue extracts from humans and animals using NMR spectroscopy for analysis of hydrophilic and hydrophobic components. As metabolomics is a fingerprint for early disease diagnostic, detected biomarkers could indicate some disease pathomechanisms.
List of cooperation centers and institutions
Clinic of Diabetology, Medical University of Warsaw, Warsaw, Poland
Clinic of Hemato-oncology and Bone Marrow Transplantation, Medical University of Lublin, Lublin Poland
1st Department of Cardiology, Medical University of Warsaw, Warsaw, Poland
II Katedra i Klinika Położnictwa i Ginekologii, Medical University of Warsaw, Warsaw, Poland
Donau University, Krems, Austria
Institute of Biochemistry and Biophysics, Polish Academy of Sciences
Mossakowski Medical Research Centre, Polish Academy of Sciences
I Department of Anaesthesiology and Intensive Care, Medical University of Warsaw, Warsaw, Poland
Departament of Neurology, Medical University of Warsaw, Warsaw, Poland
Cardiac Surgery Department, Medical University of Warsaw, Warsaw, Poland
Department of Vascular Surgery and Angiology, Medical University of Warsaw, Warsaw, Poland
Department of Neurology and Epileptology, Centre of Postgraduate Medical Education, Warsaw, Poland
Projects
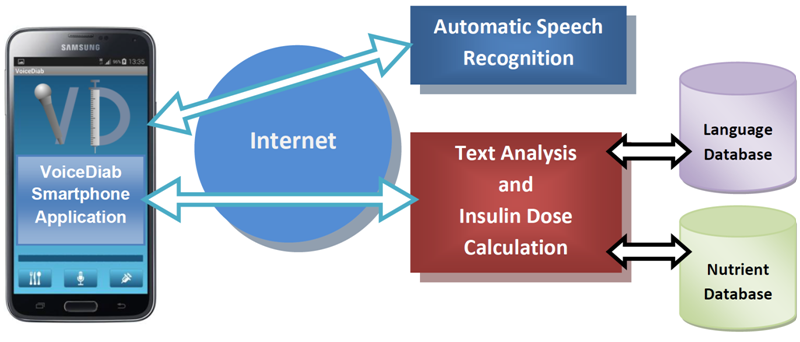
- VoiceDiab – Insulin bolus calculator with automatic speech recognition. The system calculates insulin dosage based on the verbal meal description.
Scheme of the VoiceDiab system
- metaBIAL – on-line simulator enabling comparison of efficacy of different therapy options in patients with previously untreated B-cell chronic lymphocytic leukemia in respect to the progression free survival and the overall survival (http://bco.ibb.waw.pl/en/bio-med-en/metabial-2,459/).
- NetBIAL system - Database for treatment monitoring of patients with chronic lymphocytic leukemia (http://bco.ibb.waw.pl/en/bio-med-en/netbial-2,74/).
- Model Center of Diabetes Treatment (http://mclc.ibib.waw.pl/en/index.html) with cardiologic, diabetic foot syndrome, difficult diabetes, gestational diabetes, retinopathy and education modules.
- ROTMED – Analiza profili innowacyjnych w dziedzinie inżynierii biomedycznej w Polsce (http://rotmed.ibib.waw.pl/content/project.html).
- TeleDiaFoS – Telematic system for the monitoring of patients with diabetic foot syndrome.

Patient’s module of the TeleDiaFoS system during taking a picture of foot wound (Fig. A) and data downloading from blood pressure monitor and glucometer (Fig. B)
- TeleMed – Telemedical system for supporting newly diagnosed patients with diabetes type I.
Selected papers
- Foltynski P and Ladyzynski P. Digital Planimetry With a New Adaptive Calibration Procedure Results in Accurate and Precise Wound Area Measurement at Curved Surfaces. Journal of Diabetes Science and Technology, 2020, DOI: 10.1177/1932296820959346.
- Toczylowska B, Zieminska E, Senator P, Lazarewicz JW. Hippocampal Metabolite Profiles in Two Rat Models of Autism: NMR-Based Metabolomics StudiesMol Neurobiol. 2020;57(7):3089-3105. doi: 10.1007/s12035-020-01935-0.
- Krzymien J and Ladyzynski P. Insulin in type 1 and type 2 diabetes—should the dose of insulin before a meal be based on glycemia or meal content? Nutrients, 2019;11(3):607.
- Ladyzynski P, Krzymien J, Foltynski P, Rachuta M, Bonalska B. Accuracy of Automatic Carbohydrate, Protein, Fat and Calorie Counting Based on Voice Descriptions of Meals in People with Type 1 Diabetes. Nutrients 2018;10:518. doi 10.3390/nu10040518.
- Foltynski P, Ladyzynski P, Pankowska E, Mazurczak K. Efficacy of automatic bolus calculator with automatic speech recognition in patients with type 1 diabetes: A randomized cross-over trial. J Diabetes 2018;10:600-608.doi 10.1111/1753-0407.12641.
- Foltynski P. Ways to increase precision and accuracy of wound area measurement using smart devices: Advanced app Planimator. PLOS ONE 2018;13:e0192485.
- Ciechanowska A, Ladyzynski P, Hoser G, Sabalinska S, Kawiak J, Foltynski P, Wojciechowski C, Chwojnowski A. Human endothelial cells hollow fiber membrane bioreactor as a model of the blood vessel for in vitro studies. J Artif Organs 2016;19:270-7.
- Ladyzynski P, Molik M, Foltynski P. A network meta-analysis of progression free survival and overall survival in first-line treatment of chronic lymphocytic leukemia. Cancer Treat Rev 2015;41:77-93.
- Foltynski P, Ladyzynski P, Ciechanowska A, Migalska-Musial K, Judzewicz G, Sabalinska S. Wound Area Measurement with Digital Planimetry: Improved Accuracy and Precision with Calibration Based on 2 Rulers. PLoS One 2015;10:e0134622.
- Ladyzynski P, Foltynski P, Bak MI, Sabalinska S, Krzymien J, Kawiak J. Validation of a hemoglobin A1c model in patients with type 1 and type 2 diabetes and its use to go beyond the averaged relationship of hemoglobin A1c and mean glucose level. J Transl Med 2014;12:328.
- Toczylowska B, Zieminska E, Goch G, Milej D, Gerega A, Liebert A. Neurotoxic effects of indocyanine green - cerebellar granule cell culture viability study. Biomed Opt Express 2014;5:800-16.
- Foltynski P, Ladyzynski P, Wojcicki JM. A new smartphone-based method for wound area measurement. Artif Organs 2014;38:346-52.
- Ladyżyński P, Wójcicki JM, Bąk MI, Sabalińska S, Kawiak J, Foltyński P, Krzymień J, Karnafel W. Hemoglobin glycation rate constant in non-diabetic Individuals. Ann Biomed Eng 2011;39:2721-34.
- Foltynski P, Wojcicki JM, Ladyzynski P, Migalska-Musial K, Rosinski G, Krzymien J, Karnafel W. Monitoring of diabetic foot syndrome treatment: some new perspectives. Artif Organs 2011;35:176-82.
- Ciechanowska A, Ładyżyński P, Wójcicki JM, Sabalińska S, Krzymień J, Puławska E, Karnafel W, Foltyński P, Kawiak J. Microdialysis technique as a monitoring system for acute complications of diabetes. Artif Organs 2008;32:45-52.
- (Autorzy wymienieni w kolejności alfabetycznej) Baczko T, Błażewicz S, Jasiński L, Kowalczyk M, Kowalewski T, Kędzior K, Ładyżyński P, Mączyńska E, Nowakowski A, Nowicki A, Rumiński J, Skalski K, Stodolak E, Tadeusiewicz R, Torbicz W, Wójcicki JM: System monitorowania i scenariusze rozwoju technologii medycznych w Polsce. Wójcicki J.M., Ładyżyński P. (red.), Konsorcjum ROTMED, Warszawa, 2008, 338 stron.
- Ładyżyński P, Wójcicki JM: Home telecare during intensive insulin treatment - metabolic control does not improve as much as expected. J. Telemed Telecare 2007;13:44-47.
- Ładyżyński P, Wójcicki JM, Krzymień J, Foltyński P, Migalska-Musiał K, Tracz M, Karnafel W. Mobile telecare system for intensive insulin treatment and patient education. First applications for newly diagnosed type 1 diabetic patients. Int J Artif Organs 2006;29:1074-81.